2004/9/25 朝日新聞 Science
News誌 MIT release
ホウレンソウで発電します MIT、初の光合成電池開発
http://www.asahi.com/science/update/0925/001.html
米マサチューセッツ工科大(MIT)は、ホウレンソウを使った「太陽電池」を開発したと発表した。米化学会機関誌の最新号に掲載された。発電量はまだごくわずかだが、「光合成を利用した固体電池は世界初」としている。
植物の光合成は、光のエネルギーを利用して有機物をつくる。この働きを発電に応用するアイデアは以前からあったが、光合成を担うたんぱく質を分離して使うには水分が必要で、固体電池には不向きとされてきた。研究チームは、ある種の物質を混ぜると、たんぱく質が乾燥状態でも長持ちすることを発見、入手の容易さなどからホウレンソウを材料に選んだ。
完成した電池は、ホウレンソウから抽出したたんぱく質をガラスと特殊な半導体で挟んだサンドイッチ構造。ガラス面に光を当てると、弱い電流が生じた。「電力変換効率は12%くらい。多層構造にするなどの工夫で、実用に耐えうる20%を突破したい」と研究チームは説明している。
Science News Week of June 5, 2004
Protein Power: Solar cell produces electricity from spinach and
bacterial proteins
http://www.sciencenews.org/articles/20040605/fob2.asp
Inspired by the
efficiency with which plants convert sunlight into sugar,
researchers have fabricated a solar cell that uses photosynthetic
proteins to convert light into electricity. Although the
prototype device can't yet rival commercial solar cells made of
silicon, it demonstrates a new strategy for making longer-lasting
photovoltaic cells.
 |
: |
To make the solar cell, a team of
biologists and engineers led by Marc Baldo of the
Massachusetts Institute of Technology (MIT) harvested
photosynthetic proteins from spinach and the bacterium
Rhodobacter sphaeroides and deposited the proteins onto a
glass support. Because the proteins naturally reside in
an aqueous environment inside a cell membrane, it took
some creative chemistry to keep the approximately 2
billion isolated proteins functional on a solid surface.
Consider the new material that MIT molecular biologist
Shuguang Zhang developed to stabilize the proteins. It
consists of synthetic peptides that self-assemble into
structures resembling cell membranes. When embedded in
the synthetic membranes, the photosynthetic proteins
retain their function.
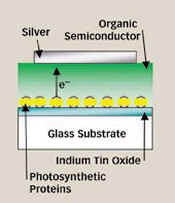
The MIT group placed a thin layer of this membrane
complex on a glass surface coated with indium tin oxide,
which served as a transparent electrode. The researchers
then added a soft layer of an organic semiconductor and
topped it all with a silver electrode. |
GREEN MACHINE.
In this prototype solar cell, photosynthetic proteins
(spheres embedded in yellow peptides) absorb light and
pump electrons (e-) into a silver electrode. |
When the
researchers shone light of certain wavelengths onto the device,
the photosynthetic proteins absorbed the photons and shunted
excited electrons through the semiconductor layer and into the
silver electrode, creating a current. Baldo and his colleagues
describe the working device in the June issue of Nano Letters.
"This is very exciting work," says Peter Peumans of
Stanford University, noting that the new strategy opens many
possibilities for making not just solar cells but also other
protein-based electronic devices. However, he says, to make a
useful solar cell, the MIT team will have to dramatically
increase the device's efficiency.
To boost the solar cell's power output, Baldo and his colleagues
are exploring ways of packing more photosynthetic proteins into
their 1-millimeter-by-1-millimeter device. One potential way of
achieving that goal is to roughen the glass to increase the
amount of surface area that can hold the proteins.
Even if Baldo and his colleagues can't boost their new solar
cell's efficiency to match that of commercial photovoltaic
devices, there could be other advantages to a protein-based
design.
For example, many solar cell materials degrade over time, but a
protein-based solar cell could be self-repairing, says Baldo.
Just as living plants replenish their photosynthetic proteins by
swapping out the old copies for new ones, it might become
possible to flush a solution of fresh proteins through a solar
cell to replace the photosynthetic molecules as they degrade,
Baldo explains.
Stephen Forrest of Princeton University says that experiments
such as Baldo's could also give researchers a greater
understanding of the mechanisms underlying photosynthesis.
"Nature has taken a very long time to optimize solar energy
collection and conversion," he says, "and it has many
strategies for doing that."
MIT September 15, 2004
Green, leafy
spinach may soon power more than Popeye's biceps
http://web.mit.edu/newsoffice/2004/spinach-0915.html
For the first
time, MIT researchers have incorporated a plant's ability to
convert sunlight to energy into a solid-state electronic “spinach sandwich” device that may one day power laptops and
cell phones. 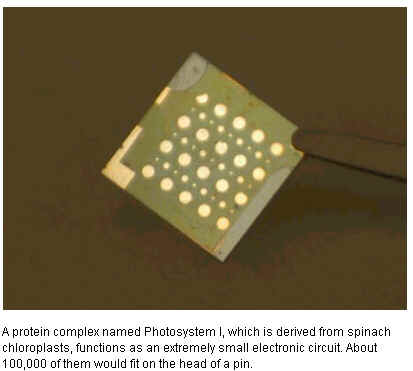
At the heart of the device is a protein complex dubbed
Photosystem I (PSI). Derived from spinach chloroplasts, PSI is 10
to 20 nanometers wide. Around 100,000 of them would fit on the
head of a pin. “They are the smallest
electronic circuits I know of,” said researcher Marc A.
Baldo, assistant professor of electronic engineering and computer
science at MIT.
Baldo and other researchers from MIT, the University of Tennessee
and the U.S. Naval Research Laboratory, including electrical and
biomedical engineers, nanotechnology experts and biologists,
collaborated on the world's first solid-state photosynthetic
solar cell. The work was reported in NanoLetters, a publication
of the American Chemical Society.
“We have crossed the first hurdle of
successfully integrating a photosynthetic protein molecular
complex with a solid-state electronic device,” Baldo said.
Plants’ ability to generate
energy has been optimized by evolution, so a spinach plant is
extremely efficient, churning out a lot of energy relative to its
size and weight. But combining biological and non-biological
materials in one device has stymied researchers in the past.
Biological materials need water and salt to survive?both are
deadly for electronics.
From wet to dry
A new twist in the current work is a membrane of peptide
surfactants - similar to the main ingredient in soap - that
helped the photosynthetic complexes self-assemble and stabilize
while the circuit was fabricated.
So far, scientists and engineers’ efforts to harness the
photosynthetic properties of green plants have been most
successful with naturally soft organic materials in liquid
solutions. But if organic solar cells are to be practical for
commercial devices, they need to be integrated with solid-state
electronics.
The researchers ground up ordinary spinach and purified it with a
centrifuge to isolate a protein deep within the cell.
The resulting dark green pellets that smell like cut grass were
purified still further and coaxed into a water-soluble state. One
of the challenges was to keep the proteins in the same
configuration as they appear naturally in the organism.
Here's where peptides come in. The 80,000-plus kinds of proteins
in our body, when in fragments called peptides, transform
themselves like tiny LEGOs? into millions of substances. Shuguang
Zhang, associate director of MIT's Center for Biomedical
Engineering, discovered that these same peptides can be tweaked
into forming completely new natural materials that perform useful
functions. One of his designer nanomaterials, which acts like the
main ingredient in soaps and detergents, turns out to be ideal
for keeping protein complexes functional on a cold, hard surface.
The spinach-sandwich device has no water. Proteins usually need
water to survive, but using Zhang's detergent peptide, the
researchers were able to stabilize the protein complexes in a dry
environment for at least three weeks. “Detergent peptide turned out to be a
wonderful material to keep proteins intact on the surface with
electronics,” Zhang said. He speculates
that the detergent material has some water trapped within it,
similar to the way plant seeds hoard oils that maintain the seeds’ integrity in dry conditions.
Building the sandwich
The bottom layer of the molecular electronic device is
transparent glass coated with a conductive material. A thin layer
of gold helps the chemical reaction that assembles the spinach
chlorophyll Photosystem I complexes. The researchers then
evaporate a soft organic semiconductor that prevents electrical
shorts and protects the protein complexes from the layer of metal
that completes the sandwich.
The researchers shone laser light on the device to create optical
excitation, then measured the resulting current. “An important caveat is that we got very
little current out, mostly because we had just a thin layer of
the complexes in our devices,” Baldo said. “Most of the optical excitation passed
straight through without being absorbed. Of the light that was
absorbed, we estimate that we converted around 12 percent to
charge.”
The researchers hope to achieve a power conversion efficiency of
20 percent or more (which would provide an extremely efficient
power source) by creating multiple layers of PSI or assembling
them on rough surfaces or 3-D surfaces, like skyscrapers that
concentrate a huge amount of surface area within a relatively
small space.
Patrick J. Kiley (S.B. 2003) of MIT also worked on this research,
which is funded by the Defense Advanced Research Projects Agency,
the Air Force Office of Scientific Research, and the National
Science Foundation.

