PERP Program - New Report
Alert
http://www.chemsystems.com/newsletters/perp/Oct03_N02S8.cfm
October 2003
Nexant's ChemSystems Process Evaluation/Research Planning program has published a new report, Developments in Non-Phosgene Polycarbonate Technology (02/03S8).
Polycarbonate demand has enjoyed extremely good growth over the last ten years driven by optical media, automotive, and glazing applications. Global capacity at the end of 2002 was about 2.6 million metric tons per year and a significant amount of new capacity has been announced. GE Plastics and Bayer are by far the two most dominant players in this market and together control nearly 60 percent of the world's polycarbonate capacity.
Early commercial production of polycarbonate was based on reacting phosgene with phenol to produce diphenyl carbonate, a high boiling liquid that was then reacted with bisphenol A to produce the polymer and liberate phenol for reuse. Later, the difficult engineering problem of achieving adequate contacting of the solid bisphenol A with gaseous phosgene was solved, leading to the dominant present day interfacial polymerization process.
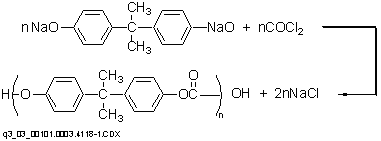
In the interfacial polymerization route, alkali salts of bisphenol A in aqueous solution are phosgenated in the presence of an inert solvent (e.g., methylene chloride) via the following reaction.

The reaction can be carried out in one or two stages (i.e. phosgenation and polycondensation) in a batch or continuous operation at 25-42ーC and near atmospheric pressure. The reactor product is a heterogeneous mixture consisting of the polymer dissolved in an organic phase, and an aqueous phase containing caustic soda, sodium salt of bisphenol A, and sodium chloride. After separation, each phase is subjected to further processing.
One of the advantages of this process is that the reaction can proceed at a low temperature in an aqueous system. Thus, no drying of starting materials is required, and the reaction is insensitive to many impurities. Polymers of high molecular weight are readily obtained. Among the operational disadvantages of this procedure are the difficulty of removing electrolytes from the polymers and the necessity of isolating polymers from relatively dilute solutions.
Non-phosgene-based plants, also called melt processes, have been commercialized by GE, Bayer, and Asahi/Chi Mei while other key Asian producers are either building non-phosgene based plants or have plans on the drawing board. The development of non-phosgene processes has been prompted by a combination of factors: health and safety concerns with the use of phosgene and methylene chloride in the conventional interfacial process, improved product quality for rapidly growing optical media applications, and last but certainly not least, the opportunity to decrease investment and operating costs for polycarbonate manufacture. A further incentive to eliminate the use of phosgene is the economic penalty incurred because the chlorine content of the phosgene is wasted and converted to sodium chloride.
All melt processes presently use the same overall approach for making polycarbonate without the use of phosgene, the transesterification of diphenyl carbonate with bisphenol A. While there are undoubtedly some engineering design differences among the various producers' transesterification technology, the significant distinguishing characteristics are the various means employed to make diphenyl carbonate and its precursors.
The preparation of aromatic polycarbonates from diphenyl carbonate and bisphenol A (BPA) by means of transesterification takes place in two stages in the absence of solvents. In the first stage, BPA and excess diphenyl carbonate are reacted and phenol is removed to produce a prepolymer.
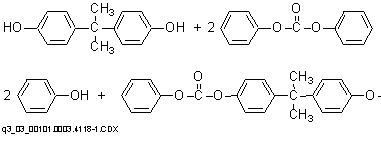
Polymerization to a high molecular weight polymer in the second stage at lower pressures (as low as 0.1 mmHg) and higher temperatures (as high as 300ーC) occurs primarily through an ester disproportionation whereby diphenyl carbonate is formed and volatilized from the system. No chain terminator is required, and molecular weight can be controlled by control of melt viscosity during the polycondensation.
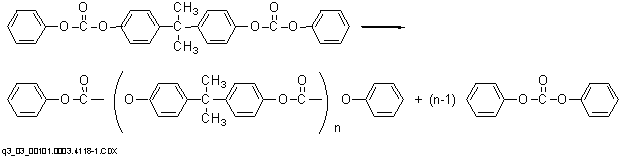
The melt process has an advantage over the commercial interfacial route in that the product is obtained in undiluted form and may be pelletized directly. Disadvantages of the process are the need for equipment capable of withstanding the use of high temperatures and high vacuum and the limitations on molecular weight imposed by the high melt viscosity.
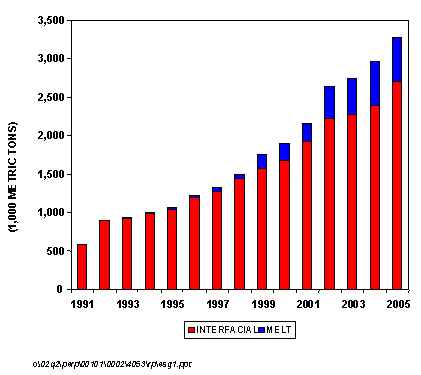
The first melt polycarbonate plant came on line in 1993 (GE Plastics), but this comprised only about 2 percent of all polycarbonate capacity. In 2001, melt plants made up about 11 percent of total capacity, and we project that by 2005, melt plants will comprise almost 17 percent of the world's polycarbonate capacity.
Non-phosgene routes presently in use make diphenyl carbonate indirectly by using an intermediate dialkyl carbonate, usually dimethyl carbonate, as the source of carbonate functionality. The first process step is the reaction of phenol with dimethyl carbonate to form the intermediate phenyl methyl carbonate in the presence of a suitable catalyst. The next step in the process involves a further reaction of the phenyl methyl carbonate with phenol or disproportionation of phenyl methyl carbonate.
Dimethyl carbonate can be made in a variety of ways:
This approach to making dimethyl carbonate was first developed by Enichem and involves a slurry phase reaction of methanol, carbon monoxide, and oxygen over a copper-based catalyst.
The chemistry of this route involves the formation of methyl nitrite from methanol, nitric oxide, and oxygen. Methyl nitrite is subsequently reacted with carbon monoxide to give dimethyl carbonate, which releases nitric oxide for recycle.
The first step is reaction of ethylene oxide with carbon dioxide to give ethylene carbonate. Catalysts that are generally known to be effective for this reaction are quaternary ammonium salts such as tetraethylammonium bromide.
In the second reaction, the ethylene carbonate is transesterified with methanol to give two products - dimethyl carbonate and ethylene glycol.
Another route to dimethyl carbonate is via the tin-catalyzed reaction of methanol with urea. A way around the problem of low yields is the use of catalytic distillation and high-boiling organic electron donor solvents. A related process involves reaction of n-butyl alcohol with urea to give di-n-butyl carbonate. Subsequent reaction of di-n-butyl carbonate with phenol gives diphenyl carbonate.
Continuing work to find improved catalysts and process conditions has progressed to the point where a direct route to diphenyl carbonate from phenol may soon challenge the indirect route through dimethyl carbonate.
Both General Electric and Bayer have extensive patents in this area, with the best results to date showing about 20 percent conversion of phenol per pass and phenol selectivities of 92-99 percent to diphenyl carbonate. However, catalyst systems involve expensive palladium and multiple other components.
In this evaluation, the oxidative carbonylation of phenol to diphenyl carbonate is assumed to take place in a series of liquid-filled, continuous stirred tank reactors operating at 90ーC and 44 psi. Total residence time in the reactors is 2 hours. The homogeneous catalyst system consists of palladium bromide, tetrabutylammonium bromide, manganese (III) acetylacetonate, and tetrabutylammonium phenolate.
Phenol conversion over all reactor stages is 60 percent. Selectivity of phenol to diphenyl carbonate is 94.5 mole percent. Diphenyl carbonate is recovered and purified by distillation.
Resins of similar viscosity produced by interfacial and melt processes behave the same in typical processing and fabrication procedures, although melt resin has a wider MW range than interfacial resin. Melt-produced resin is extremely suitable for optical grades in terms of transparency and melt flow.
The melt process presently has process and grade limitations, though it is estimated that the melt process eventually will be able to produce about 90 percent of existing interfacial grades. When the resin is subjected to multiple heat histories (extended time spent at high temperatures), melt process polycarbonate may be at an economic disadvantage, since its lower thermal stability would prevent or limit scrap reuse.
Polycarbonate (PC) is an amorphous, clear polymer with high transparency, superior dimensional stability, good electrical properties, good thermal stability, inherent fire resistance, and outstanding impact strength and ductility. Bisphenol A polycarbonate was the first major amorphous engineering plastic commercialized. It now trails only crystalline nylon in production volume within the engineering polymer category.
Key advantages and disadvantages of polycarbonate resins are listed in the following table.
Key Advantages and
Disadvantages of Polycarbonates
| Advantages | Disadvantages |
| Impact resistance | Solvent resistance |
| Ductility | Abrasion resistance |
| Clarity | Limited UV resistance |
| Dimensional stability | Notch sensitivity |
| Creep resistance | Limited hydrolytic stability |
| Rigidity | |
| High temperature resistance | |
| Electrical properties | |
| Inherent fire resistance | |
| Food compatibility |
PC has good resistance to UV light, but may experience some yellowing with long exposure. The UV resistance can be improved with UV absorbers, coatings, or coextruded layers. Coatings or coextruded layers are also used to offset PC's limited chemical and scratch resistance. The notch sensitivity of PC can be improved by adding impact modifiers or by blending with other polymers.
Polycarbonate has good resistance to a wide variety of foodstuffs and a great degree of resistance to staining by such products as tea and coffee. The rate of oxygen or carbon dioxide transmission through polycarbonate is relatively high, which is desirable for certain packaging applications and unsuitable for others.
Polycarbonate resins are available in a range of melt viscosity grades, as determined by molecular weight. Melt flow rate, rather than molecular weight, is typically used to define polycarbonate grades, with standard commercial grades available having melt flows of 4 to 22 grams per ten minutes. Special high-flow grades are used for optical disc applications (MFR = 80).
Polycarbonate has an energy absorption per unit volume of about 8.5 times higher than the energy absorption of cast aluminum, and about 60 percent that of carbon steel. Weight savings as a metal replacement are substantial, because polycarbonate is only about 40 percent as dense as aluminum and about one sixth as dense as steel.
Impact resistance 250 times greater than glass and 30 times greater than acrylic makes polycarbonate the natural choice for window replacement where breakage is common (trains, buses, schools, etc.). For security applications, polycarbonate offers unsurpassed projectile-stopping capability, as the material softens upon impact with a bullet, absorbing the projectile's energy.
Reinforced and filled grades are available. Typical grades contain from 5 to 45 percent glass fiber reinforcement. Copolymers incorporating tetramethyl bisphenol A, terephthalic acid, or organosilicones are a means of modifying the characteristics of standard bisphenol A polycarbonate. Blends of polycarbonate with polybutylene terephthalate or acrylonitrile-butadiene-styrene polymers are a commercially important means of attaining the desired properties for particular market segments, such as automotive (PBT) and machine/appliance housings (ABS).
Additional application areas for polycarbonate include medical devices, protective gear for work and sports, electrical connectors, exterior lighting, prescription lenses, and graphic arts (film).
This report discusses the process technology involved in the melt polycarbonate process, including precursor diphenyl carbonate. Comparative information is presented for the dominant interfacial process.
Cost of production estimates are provided for diphenyl carbonate via indirect (dimethyl carbonate precursor) and direct oxidative carbonylation routes. Polycarbonate production economics are also developed for melt PC with DPC from each of these sources, and for interfacial process PC.
Supply and demand estimates for PC in the United States, Western Europe, and Japan extend through 2010.
PERP
Program - New Report Alert
August 2002
Chem Systems' Process Evaluation/Research Planning program has published a new report, Polycarbonates (01/02-5).
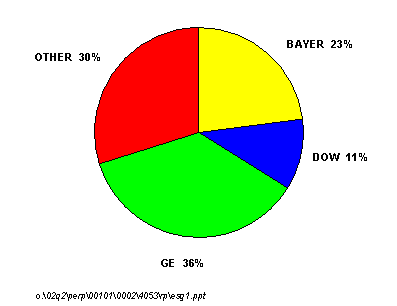
Polycarbonate demand has enjoyed extremely good growth over the last ten years driven by optical media, automotive, and glazing applications. Global capacity at the end of 2001 was 2.2 million metric tons per year and a significant amount of new capacity has been announced. GE Plastics and Bayer are by far the two most dominant players in this market and together control nearly 60 percent of the world's polycarbonate capacity. Dow Chemical is the next largest producer with an 11 percent share of total capacity.
GLOBAL POLYCARBONATE CAPACITY SHARE, 2002
Technology advances are driving change in this industry segment. Non-phosgene-based plants, also called melt processes, have been commercialized by GE and Bayer while some of the key Asian producers are either building non-phosgene based plants or have plans on the drawing board. The development of non-phosgene processes has been prompted by a combination of factors: health and safety concerns with the use of phosgene in the conventional interfacial process, improved product quality for rapidly growing optical media applications, and last but certainly not least, the opportunity to decrease investment and operating costs for polycarbonate manufacture.
As a measure of the growing importance of the non-phosgene or melt processes, the figure below shows the overall growth in polycarbonate capacity for both conventional interfacial and non-phosgene melt technologies from 1991 to 2005. As can be seen, the first melt polycarbonate plant came on line in 1993 (GE Plastics), but this comprised only about 2 percent of all polycarbonate capacity. In 2001, melt plants made up about 11 percent of total capacity and we project that by 2005, melt plants will comprise almost 17 percent of the world's polycarbonate capacity. This new report from Nexant/Chem Systems discusses the various issues and approaches surrounding this new technology. Process economics of conventional interfacial technology is compared to the newer non-phosgene routes.
PC GLOBAL CAPACITY GROWTH BY TECHNOLOGY