トップページ
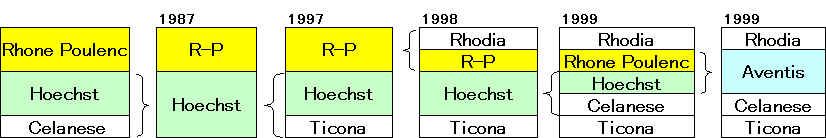
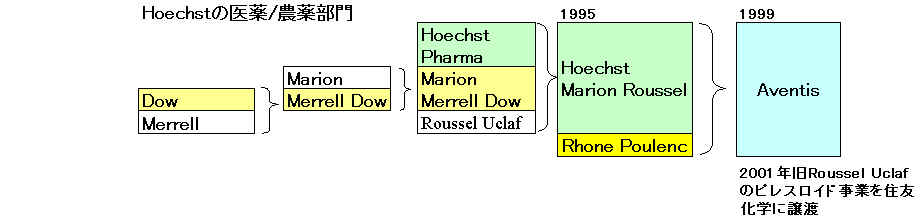
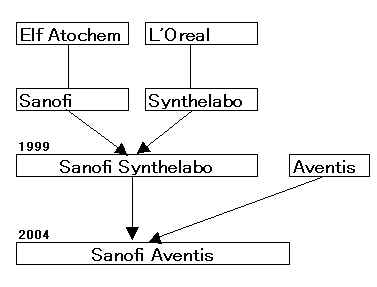
Aventis
Sanofi-Synthelabo
サノフイ
アベンティス買収へ 7兆円で合意
Aventis
Supervisory Board Recommends Substantially Improved Offer
from Sanofi-Synthelabo
「サノフィ・アベンティス」 2004/8/20に正式発足
Is
Sanofi-Aventis After Bristol-Myers?
Sanofi
Aventi
Sanofi-aventis Announces
Non-Binding Offer to Acquire Genzyme
2004/09/03 グラクソ・スミスクライン
グラクソ・スミスクライン、サノフィ・アベンティスより
抗血栓症剤 フラキシパリン、アリクストラおよび関連生産施設の買収を完了
http://release.nikkei.co.jp/detail.cfm?relID=80418
2004年9月1日 ロンドン発−グラクソ・スミスクライン(以下、GSK)は、サノフィ・アベンティス社より注射剤の抗血栓症剤 フラキシパリンとアリクストラおよび関連資産(フランスのノートルダム・ド・ボンドビルにある製造施設を含む)について世界規模での買収が成功裏に完了したことを発表しました。
この買収において、GSKは現在進行しているアリクストラの臨床試験の実施の責任も担います。
サノフィ・サンテラボ社によるアベンティス社の買収は、統合における必須条件を処理し欧州委員会および米国連邦公正取引委員会からの承認を得て完了しました。この度の製品の買収はそれにともない成立に至ったものです。
(以下略)
BusinessWeek
January 29, 2007
Word is the two
major pharmaceuticals may soon merge and become the world's
largest drugmaker ahead of some best-selling drugs coming off
patent
Speculation is mounting
that the long-rumored merger between Franco-German drugmaker
Sanofi-Aventis and New York-based Bristol-Myers Squibb may soon
be reality. If true, the deal will enable Sanofi to vault past
Pfizer and Britain's GlaxoSmithKline to become the world's
largest drugmaker, with combined annual revenues of nearly $55
billion. Neither company is commenting. But a recent report in
French financial newsletter La Lettre de
l'Expansion claims the two companies have
already signed a pre-merger agreement.
December 15, 2015
Sanofi and Boehringer Ingelheim Enter Exclusive Negotiations on Business Swap
Sanofi Would Become a Global Leader in
Consumer Healthcare and Boehringer Ingelheim Would Become Second largest
Animal Health Company
Sanofi and Boehringer Ingelheim today
announced that the companies have entered into exclusive negotiations to swap
businesses.
The proposed transaction would consist of an exchange of
Sanofi animal health business ("Merial") with an enterprise value of €11.4 bn
and
Boehringer Ingelheim consumer healthcare (CHC) business with an enterprise value
of €6.7 bn.
Boehringer Ingelheim CHC business in China would be excluded from the
transaction.
The transaction would also include a gross cash payment from Boehringer
Ingelheim to Sanofi of €4.7 bn.
The transaction would allow Sanofi to become the number one ranked player in
consumer healthcare with expected pro forma sales of approximately €5.1 bn in
2015(e) and a global market share close to 4.6%1. Sales of Boehringer Ingelheim
CHC business (excluding China) are estimated at about €1.6 bn for 2015 and are
highly complementary with those of Sanofi CHC, both in terms of products and
geographies.
Boehringer Ingelheim CHC would improve the position of Sanofi in Germany and
Japan where Sanofi CHC presence is limited, and expand Sanofi presence in its
Priority Categories. Sanofi would gain access to iconic brands in
Antispasmodics, Gastrointestinal, VMS and Analgesics, and attain critical mass
in Cough & Cold. Sanofi CHC business in the US, Europe, Latin America and
Eurasia would also expand significantly, giving it multiple leadership postions
in key countries and/or on key product categories.
The animal health industry is a very attractive industry in terms of innovation,
growth potential and profitability. Combining Merial's and Boehringer
Ingelheim's complementary strengths would create the second largest player in
the global animal health market with pro forma sales of approximately € 3.8 bn
in 2015(e) with the ability to compete for global market leadership. The
combined portfolios and technology platforms in anti-parasitics, vaccines and
pharmaceutical specialities would place the combined company in the key growth
segments of the industry. The species portfolios are highly complementary
building on Merial's expertise in companion animals and poultry and BI's
expertise in swine.
"In entering into exclusive negotiations with Boehringer Ingelheim, we have
acted swiftly to meet one of the key strategic objectives of our roadmap 2020,
namely to build competitive positions in areas where we can achieve leadership.
This transaction would allow Sanofi to become a world leader in the attractive
non-prescription medicines market and would bring a complementary portfolio with
highly recognized brands, allowing for mid and long term value creation," said
Olivier Brandicourt, M.D., Chief Executive Officer, Sanofi. "I am confident that
Boehringer Ingelheim will enable Merial to fully express and develop its
potential in the attractive but competitive animal health market."
Germany would become a key center of Sanofi CHC business, including in
particular for Gastro-Intestinal and Cough & Cold categories that will benefit
from the strong capabilities of Boehringer Ingelheim teams. Sanofi will pay
particular attention to social matters as well as skills and people retention
sensitivities.
"Boehringer Ingelheim's strategic priority is to focus on the company's core
areas of expertise and businesses with an established global scale, or where a
pathway to a global scale can be achieved and prioritized among Boehringer
Ingelheim's portfolio opportunities," said Prof. Dr Andreas Barner, Chairman of
the Board, Boehringer Ingelheim. "Boehringer Ingelheim Animal Health is and will
stay strongly committed to bringing novel, innovation driven solutions to
veterinarians and animal owners. Our combined Animal Health business would be
well positioned for growth and emergence as a leader globally. I am confident
that Sanofi will enable our CHC business to fully live its potential supported
by highly professional and committed teams."
Lyon would be a key operational center of Boehringer Ingelheim's Animal Health
business. Boehringer Ingelheim will commit to maintain business operations, R&D
and Manufacturing Centers in France. As the U.S. market is an important part of
Merial's business, Boehringer Ingelheim would pay particular attention to
sustain the momentum of the U.S. operations. Boehringer Ingelheim will give
particular attention to social matters as well as skills and retention
sensitivities.
The execution of definitive agreements is expected in the coming months
following consultations with the relevant social bodies. Boehringer Ingelheim
and Sanofi's goal currently is to close the potential transaction in Q4 2016,
subject to appropriate regulatory approvals.
Sanofi intends to use a portion of the net proceeds of the transaction to
repurchase shares. Taking into account the anticipated CHC results, share
buybacks and potential synergies, the overall transaction is expected to be
business EPS neutral in 2017 and accretive in subsequent years.
About Boehringer Ingelheim's CHC activities
Boehringer Ingelheim Consumer Healthcare is the 8th largest Consumer Healthcare
business in the world. Sales of Boehringer Ingelheim's CHC business were €1.4 bn
in 2014, contributing 11 per cent to Boehringer Ingelheim's net sales. It aims
to create and drive inspiring and sustainable solutions that will efficiently
develop compelling and true global brands for consumers.The leading brands of
Boehringer Ingelheim's CHC business are the antispasmodic Buscopan® (2014 sales
of €219 million; mainly sold in Europe and Latin America), the laxative Dulcolax®
(2014 sales of €204 million; sold in more than 40 countries with a strong
presence in the U.S.), the multivitamins Pharmaton® (2014 sales of €133 million,
with majority of sales in Latin America), the cough treatments Mucosolvan® (2014
sales of €165 million, mainly in China, Germany and Russia) and Bisolvon® (2014
sales of €101 million with a fragmented worldwide presence with largest
countries being Spain and Italy) and the cold treatment Mucoangin®/Lysopaine®
(2014 sales of €48 million).
About Sanofi CHC activities
Sales of Sanofi CHC business were €3.3 bn in 2014. The leading brands of
Sanofi's CHC business are the allergy products Allegra® (2014 sales of €350
million) and Nasacort® (2014 sales of €114 million), the pain killers Doliprane®
(2014 sales of €310 million), No-Spa® (2014 sales of €109 million) and Dorflex®
(2014 sales of €90 million), the digestive products Essentiale® (2014 sales of
€235 million), Enterogermina® (2014 sales of €156 million) and Maalox® (2014
sales of €98 million), the feminine care product Lactacyd® (2014 sales of €104
million) and the vitamins, minerals and supplements Magné B6® (2014 sales of €88
million). In 2014, 52.6% of CHC sales were generated in emerging markets, 21.2%
in the U.S. and 20.3% in Western Europe.
Sanofi and Boehringer Ingelheim combined worldwide CHC businesses - Priority
Categories
(pro forma)
Source: Nicholas Hall & Company, FY 2014
| Global Categories*
|
Segment size in €bn |
Sanofi + BI CHC |
| Pain Care
|
13.2 |
#2 |
| Allergy Solutions
|
3.1 |
#3 |
| Cough & Cold Care
|
17.2 |
#6 |
| Feminine Care
|
0.8 |
#1 |
| Digestive health |
14.4
|
#1 |
| ビタミン・ミネラル・サプリメント VMS2
|
27.6 |
#3 |
About Merial
Merial is a world-leading, innovation-driven animal health company, providing a
comprehensive range of products to enhance the health and well-being of a wide
range of animals. Merial employs 6,600 people and operates in more than 150
countries worldwide with €2.5 billion of sales expected in 2015.
Merial has three main business areas: pets, farm animals, and veterinary public
health. Our pharmaceuticals and vaccines focus on disease prevention and overall
health and wellness in animals, and target more than 200 diseases and conditions
across a variety of species.
The main Merial brands in the field of pet health include Frontline®, Heartgard®,
NexGard® and Purevax®; and in farm animals Vaxxitek®, Eprinex®, Ivomec®,
Longrange®, Circovac® and GastroGard®. Merial is a strategic partner for
veterinary public health (VPH), providing governments with vaccines to control
foot and mouth disease (FMD), Rabies and Blue Tongue Virus (BTV).
As part of our commitment to innovation and excellence, Merial has a global
footprint with 13 R&D centers and 18 production sites. Working with global
trends in mind, such as population growth, increased demand for animal proteins,
needs of emerging markets, growing middle classes, resource constraints, and
sustainability concerns, we aim to consciously and constantly innovate and
provide solutions to our customers' and animals' needs.
For more information, please see www.merial.com
About Boehringer Ingelheim Animal Health
Boehringer Ingelheim Animal Health is the 6th largest animal health business in
the world and is committed to providing leading innovative solutions to prevent,
treat and cure animal diseases. With approximately 4,000 employees worldwide,
Boehringer Ingelheim Animal Health achieved net sales of about € 1.13 billion in
2014. Main brands of Boehringer Ingelheim's Animal Health portfolio are Ingelvac
Circoflex® (2014 sales of € 260 million), Metacam® (2014 sales of € 93 million),
Ingelvac PRRS® (2014 sales of € 74 million), Duramune® (2014 sales of € 69
million) and Vetmedin® (2014 sales of € 57 million), representing 8% of the
corporation's revenue base. From a regional perspective, Boehringer Ingelheim
has its legacy as an Animal Health company in the US with most of its R&D and
manufacturing infrastructure located there. Significant investments have been
made recently in Hannover, Germany and are underway in China. The Boehringer
Ingelheim Animal Health Business is currently holding strong positions in swine
and cattle vaccines as well as in pharmaceutical specialties for animal use. In
its research-driven Animal Health business, Boehringer Ingelheim continually
invests more than 12% of net sales of the Animal Health business in R&D.
Boehringer Ingelheim and Merial combined businesses - priority categories (pro
forma)
| Global Categories |
Global segment size
in €bn |
Boehringer Ingelheim + Merial |
| Small Animals
|
7,1 |
#1 |
| Ruminants 反芻動物 |
5,8
|
#4 |
| Swine
豚 |
2,6 |
#1 |
| Poultry
家禽 |
2,4 |
#3 |
| Equine
馬 |
0,6 |
#1 |
About Sanofi
Sanofi, a global healthcare leader, discovers, develops and distributes
therapeutic solutions focused on patients' needs. Sanofi has core strengths in
diabetes solutions, human vaccines, innovative drugs, consumer healthcare,
emerging markets, animal health and Genzyme. Sanofi is listed in Paris (EURONEXT:
SAN) and in New York (NYSE: SNY).
About Boehringer Ingelheim
The Boehringer Ingelheim group is one of the world's 20 leading pharmaceutical
companies. Headquartered in Ingelheim, Germany, Boehringer Ingelheim operates
globally with 146 affiliates and a total of more than 47,700 employees. The
focus of the family-owned company, founded in 1885, is researching, developing,
manufacturing and marketing new medications of high therapeutic value for human
and veterinary medicine.
Social responsibility is an important element of the corporate culture at
Boehringer Ingelheim. This includes worldwide involvement in social projects,
such as the initiative "Making more Health" and caring for the employees.
Respect, equal opportunities and reconciling career and family form the
foundation of the mutual cooperation. In everything it does, the company focuses
on environmental protection and sustainability.
In 2014, Boehringer Ingelheim achieved net sales of about 13.3 billion euros.
R&D expenditure corresponds to 19.9 per cent of its net sales.
2020年4月14日
2020/6 第1/2相試験を2020年9月に開始する計画 別途、サノフィは
Translate Bio と提携(下記)
COVID-19ワクチンの開発に向けて、サノフィとGSKが前例のない提携を開始
-
両社の技術を組み合わせ、アジュバント添加COVID-19ワクチンの開発を目指す
-
2020年下半期に候補ワクチンの臨床試験を開始する予定であり、成功すれば、2021年下半期に実用化の見込み
サノフィ(本社:フランス)とグラクソ・スミスクライン(本社:英国、GSK)は本日、現在の新型コロナウイルスによる感染症の世界的大流行に対処するため、両社の技術を活かし、COVID-19に対するアジュバント添加ワクチンを開発する同意書に署名したことを発表しました。
サノフィは、遺伝子組換えDNA技術をベースとするS-タンパク質COVID-19抗原を提供します。遺伝子組換えDNA技術により、ウイルスの表面に検出されたタンパク質と正確に一致する遺伝子配列を作成することができます。抗原をコード化するDNA配列を、サノフィが米国で開発に成功し承認された遺伝子組換えインフルエンザワクチンの基盤となったバキュロウイルス発現プラットフォームに組み込みます。
GSKは、実証済みのパンデミックアジュバント技術を提供します。アジュバントの使用はパンデミックの状況下では特に重要です。アジュバントを使用することにより、1回の接種に必要なワクチン用タンパク質の量が抑えられるため、ワクチンの生産量を増やすことができ、より多くの人々を守ることに貢献できるからです。
サノフィ最高経営責任者(CEO)のポール・ハドソンは、次のように述べています。「世界は未曾有の医療危機に直面しており、1つの企業が単独で対処することは不可能です。そのため、サノフィは、GSKなどの業界他社と協力し自社の専門知識とリソースを補完することで、十分な数量のワクチンを作り出して供給するという目標に向けて取り組んでいます。」
GSK最高経営責任者(CEO)のエマ・ウォルムズリーは、次のように述べています。「この提携により、世界トップクラスのワクチン企業2社が協力することになります。両社の科学的専門知識、技術、能力を組み合わせることにより、ワクチンを開発する世界的な取り組みを加速し、できるだけ多くの人々をCOVID-19から守りたいと考えています。」
タンパク質ベースの抗原をアジュバントと組み合わせる方法は、現在提供されている多くのワクチンで使用されている確立された方法です。アジュバントは、免疫応答を高めるために一部のワクチンに添加するもので、それによって感染症に対し、ワクチン単体よりも、強力かつ長期的に持続する免疫を作り出すことができます。また、有効なワクチンを大量に生産できる可能性も高まります。
両社は、2020年下半期に第I相臨床試験を開始する予定であり、これに成功すれば、規制当局による審査を経て、2021年下半期までに実用化できるよう、開発の完了を目指します。
これまでにサノフィが公表したとおり、遺伝子組換え技術をベースとするCOVID-19ワクチン候補の開発は、米国保健福祉省(HSS)事前準備・対応担当次官補局(ASPR)の一部門である米国生物医学先端研究開発局(BARDA)との協力の下で、同局の資金提供を受けて実施されています。両社は、他の政府や世界的なアクセスを優先する国際機関からの財政支援について協議を進める予定です。
BARDAのディレクターであるリック・A・ブライト博士(Rick
A. Bright, Ph.D)は次のように述べています。「コロナウイルスのワクチンをできるだけ早く提供するには、ワクチン業界のリーディングカンパニーによる戦略的提携が不可欠です。COVID-19に対するアジュバント添加遺伝子組換えワクチン候補を開発すれば、ワクチンの用量を減らし、より多くの人々にワクチンを提供しこのパンデミックを収束させ、将来的なコロナウイルスの流行に適切に備えるだけでなく、予防することも可能になるでしょう。」
両社は、サノフィのワクチン事業部門のグローバルヘッドであるデヴィッド・ロウと、GSK グローバルワクチン
プレジデントのロジャー・コナーが共同議長を務める共同タスクフォースを設置しました。タスクフォースは、両社のリソースを動員し、ワクチン候補の開発を加速するあらゆる機会を模索していきます。
パンデミックには大きな人的・資金的課題が伴うことを考慮し、両社はCOVID-19ワクチンが世界中で使用できるようにすることを最優先し、すべての国の人々に対して、公正に提供できる仕組みを通じて、一般の人々がこの提携によって開発されたワクチンを使用できるよう努めていきます。
今回の提携は、COVID-19との闘いにおいてサノフィとGSKが現在取り組んでいる活動の中で、重要な節目となります。両社は、研究成果有体物移転契約(MTA)を締結し、すみやかに共同研究を進められるようにしました。提携の最終的な条件は、今後数週間以内に確定する予定です。
サノフィについて
サノフィは、健康上の課題に立ち向かう人々を支えます。私たちは、人々の健康にフォーカスしたグローバルなバイオ医薬品企業として、ワクチンで人々を守り、革新的な医薬品で痛みや苦しみを和らげます。希少疾患をもつ少数の人々から、慢性疾患をもつ何百万もの人々まで、寄り添い支え続けます。サノフィでは、100カ国において10万人以上の社員が、革新的な医科学研究に基づいたヘルスケア・ソリューションの創出に、世界中で取り組んでいます。
サノフィは、「Empowering
Life」のスローガンの下、ヘルスジャーニー・パートナーとして人々を支えます。日本法人であるサノフィ株式会社の詳細は、http://www.sanofi.co.jpをご参照ください。
June 23 2020
Sanofi and Translate Bio expand collaboration
to develop mRNA vaccines across all infectious disease areas
The two companies will build upon their
existing collaboration to pursue novel mRNA vaccines aimed at broadly
addressing current and future infectious diseases
Translate Bio to receive $425 million in upfront payment and common stock
equity investment and overall is eligible to receive up to $1.9 billion of
potential milestones/payments as well as tiered royalties on worldwide sales
of developed vaccines
Sanofi to receive exclusive worldwide
rights to develop, manufacture and commercialize infectious disease vaccines
using Translate Bio technology
The expanded collaboration brings together Translate Bio’s leading mRNA
technology and manufacturing with Sanofi’s world class vaccine development
and distribution
Sanofi Pasteur, the vaccines global business
unit of Sanofi, and Translate Bio, a clinical-stage messenger RNA (mRNA)
therapeutics company, have agreed to expand their existing
2018 collaboration and license agreement to develop
mRNA vaccines for infectious diseases.
The expansion of this agreement will further unite Translate Bio’s expertise and
knowledge from more than 10 years of mRNA research and development with Sanofi’s
leadership in vaccine research and development.
Under the expansion agreement, Translate Bio will receive
a total upfront payment of $425 million, consisting of a
$300 million cash payment and
a private placement common stock investment of $125 million at $25.59 per
share representing a 50 percent premium to the 20-day moving average share price
prior to signing.
Translate Bio will also be eligible for
potential future milestones and other payments up to $1.9
billion, including $450 million of milestones under the 2018 agreement.
Of these potential milestones and other payments, approximately $360 million are
anticipated over the next several years, inclusive of COVID-19 vaccine
development milestones. In addition, Translate Bio is also eligible to receive
tiered royalty payments based upon worldwide sales of the developed vaccines.
Sanofi Pasteur will pay for all costs during the collaboration term. Under this
agreement Sanofi Pasteur will receive exclusive worldwide rights for infectious
disease vaccines.
“As all eyes are on prevention of infectious disease through vaccines, this is a
pointed moment in time where we are called upon to seek innovative ways to
protect public health,” said Thomas Triomphe, Executive Vice President, Sanofi
Pasteur. “We are excited by the novel technology and expertise Translate Bio
brings, and we believe that adding this mRNA platform to our vaccines
development capabilities will help us advance prevention against current and
future infectious diseases.”
“The expansion of our collaboration with Sanofi Pasteur validates the progress
we’ve made in the development of mRNA vaccines for infectious diseases since our
work together began in 2018 and also speaks to the potential of our mRNA
platform. We are excited to work with Sanofi in this broadened capacity with the
goal of ultimately delivering vaccines on a global scale, a need underscored by
the current pandemic,” said Ronald Renaud, Chief Executive Officer of Translate
Bio. “Translate Bio will also be well positioned financially to continue to
build upon our internal capabilities with a focus on advancing innovations in
platform discovery and on the development of ongoing and additional preclinical
therapeutic programs as we aim to bring multiple programs towards clinical
development.”
Under the collaboration, Translate Bio is using its mRNA platform to discover,
design and manufacture vaccine candidates and Sanofi Pasteur is providing its
deep vaccine expertise to advance vaccine candidates into and through further
development. Translate Bio will also transfer technology
and processes to allow Sanofi Pasteur to develop and manufacture mRNA vaccines
for infectious diseases.
The teams are currently evaluating multiple COVID-19 vaccine candidates in vivo
for immunogenicity and neutralizing antibody activity to support lead candidate
selection and the companies have the goal of initiating a first-in-human
clinical trial in the fourth quarter of 2020.
The companies are also advancing an mRNA vaccine development candidate against
influenza through preclinical studies with clinical trial initiation anticipated
in mid-year 2021. Additional mRNA vaccine development programs under the
collaboration include another viral pathogen and a bacterial pathogen.
The transactions, including the equity sale, are subject to customary closing
conditions, including termination or expiration of any applicable waiting
periods under the Hart-Scott-Rodino Act. For more information regarding the
financial and other terms of the agreement, please refer to the Current Report
on Form 8-K which will be filed by Translate Bio with the U.S. Securities &
Exchange Commission on June 23, 2020.
Evercore acted as financial advisor to Translate Bio for the expansion of the
collaboration agreement.
About mRNA Vaccines
Vaccines work by mimicking disease agents to stimulate the immune system;
building up a defense mechanism that remains active in the body to fight future
infections. mRNA vaccines offer an innovative approach by delivering a
nucleotide sequence encoding the antigen or antigens selected for their high
potential to induce a protective immune response. mRNA vaccines also represent a
potentially innovative alternative to conventional vaccine approaches for
several reasons - their high potency, ability to initiate protein production
without the need for nuclear entry, capacity for rapid development and potential
for low-cost manufacture and safe administration using non-viral delivery. This
approach potentially enables the development of vaccines for disease areas where
vaccination is not a viable option today. Additionally, a desired antigen or
multiple antigens can be expressed from mRNA without the need to adjust the
production process offering maximum flexibility and efficiency in development.
About the Sanofi Pasteur and Translate Bio collaboration
In 2018, Translate Bio entered into a collaboration and exclusive license
agreement with Sanofi Pasteur Inc., the vaccines global business unit of Sanofi,
to develop mRNA vaccines for up to five infectious disease pathogens. This
agreement was first expanded in March 2020 to include the collaborative
development of a novel mRNA vaccine for COVID-19. This collaboration brings
together Sanofi Pasteur’s leadership in vaccines and Translate Bio’s mRNA
research and development expertise. Under the agreement, the companies are
jointly conducting research and development activities to advance mRNA vaccines
and mRNA vaccine platform development during a research term of at least four
years after the original signing in 2018. Translate Bio and Sanofi Pasteur have
advanced the preclinical development vaccine programs including screening,
optimization and production of mRNA and LNP formulations across multiple
targets.
About Translate Bio
Translate Bio is a clinical-stage mRNA therapeutics company developing a new
class of potentially transformative medicines to treat diseases caused by
protein or gene dysfunction. Translate Bio is primarily focused on applying its
technology to treat pulmonary diseases caused by insufficient protein production
or where the reduction of proteins can modify disease. Translate Bio’s lead mRNA
therapeutic program is being developed as a treatment for cystic fibrosis (CF)
and is in a Phase 1/2 clinical trial. The Company also believes its technology
is applicable to a broad range of diseases, including diseases that affect the
liver. Additionally, the platform may be applied to various classes of
treatments, such as therapeutic antibodies or vaccines in areas such as
infectious disease and oncology. For more information about the Company, please
visit www.translate.bio or on Twitter at @TranslateBio
Sanofi to acquire
Principia Biopharma
・Further
strengthens core R&D areas of autoimmune and
allergic
diseases
・Provides
full control of brain-penetrant BTK
inhibitor SAR442168 in
multiple sclerosis (MS), making commercialization more efficient and
eliminating future royalty payments
・Allows
expansion of SAR442168 development program into other
central nervous system diseases and therapeutic areas
・Adds
clinically
advanced oral BTK inhibitor rilzabrutinib
with
potential
across a range of immunology and inflammation indications,
complementing Sanofi’s
existing R&D pipeline
Principia Biopharma
Inc.は臨床段階のバイオ医薬品会社である。
【事業内容】同社は免疫・腫瘍学分野において患者に対する経口療法の開発に焦点を当てる。同社はテーラード・コバレント・プラットフォームを使用して、可逆的な共有結合および不可逆的な共有結合性小分子阻害剤を設計・開発する。同社のパイプラインはPRN1008、PRN1371およびPRN2246を含む。PRN1008薬物候補は慢性免疫学的障害の治療に設計される。PRN2246は多発性硬化症(MS)および他の中枢神経系(CNS)疾患の治療用不可逆的共有結合BTK阻害剤である。PRN1008はまた、天疱瘡などの複数の自己免疫疾患の治療に開発される。PRN1371は、固形腫瘍の治療に設計された線維芽細胞増殖因子受容体(FGFR)の阻害剤である。同社はまた、炎症および自己免疫疾患の治療用経口免疫プロテアソーム阻害剤の開発に焦点を当てる。
2017/11
Principia Biopharma has signed a deal
worth up to $765 million with Sanofi for the development of a multiple
sclerosis drug candidate.
Sanofi will develop privately-held Principia’s Bruton's tyrosine kinase
(BTK) inhibitor PRN2246(SAR442168),
an experimental oral treatment for MS. The drug is designed to access
the brain and spinal cord by crossing the blood-brain barrier and impact
immune cell and brain cell signaling. Through this mechanism of action,
the company believes it has the potential to treat MS as well as other
diseases of the central nervous system. Principia said it has initiated
a Phase I trial for PRN2246 in healthy volunteers.
2020/2 サノフィがPrincipiaから導入したBTK阻害剤SAR442168による多発性硬化症(MS)治療の第2相試験が、主要評価項目である脳のMRI病変減少を達成
今回、Principia Biopharmaを買収し、SAR442168
をfull control へ
Sanofi
and
Principia Biopharma
Inc.,
a
late-stage
biopharmaceutical
company
focused
on
developing
treatments for immune-mediated
diseases,
entered into a definitive agreement
under which Sanofi will acquire all of the
outstanding
shares of
Principia
for $100 per
share
in cash, which represents an aggregate equity
value of
approximately $3.68billion
(on a
fully diluted basis).
The
Sanofi and
PrincipiaBoards
of Directors unanimously approved
the transaction.
“This acquisition advances our ongoing R&D transformation to accelerate
development of the most promising medicines that will address significant
patient
needs,”
said Paul Hudson, Sanofi Chief Executive
Officer.
“The addition of
multiple
BTK inhibitors
to our pipeline
demonstrates our commitment to strategic product
acquisitions in our
priority therapeutic areas. Full
ownership of
our brain-penetrant
BTK inhibitor ‘168
removes
complexities for this priority development program and
simplifies future commercialization.”
“The
Phase 2b data in
relapsing multiple sclerosis showed the
strong potential of
‘168 to address disability and disease progression, and triggered
the start of
Phase
3 studies
across the full spectrum of MS.
Through this acquisition, we will be able
to expand and accelerate development
of
BTK inhibitors
across multiple
indications.
Both‘168
and rilzabrutinib, have
‘pipeline in a product’ potential, and
we look forward to
unlocking their
full treatment
benefits
across
an array of
diseases,”
said John Reed, M.D., Ph.D., Global Head of Research & Development
at Sanofi.
“Principia’s
successful design and development
of a whole portfolio of BTK
inhibitors for immunology is aimed to transform the treatment for patients with
immune-mediated
diseases. By combining with Sanofi, we will bring significant
resources to expand and accelerate the potential benefits of these therapies.
The
benefit of developing several BTK inhibitors will allow us to target specific
organ
systems for optimal patient benefit. The merger will provide global resources to
get
these novel therapies to patients faster,” said
Martin Babler,
President
and CEO at
Principia Biopharma.
Principia’s
Bruton
tyrosine kinase (BTK) inhibitors
add to Sanofi’s efforts to accelerate and
build a portfolio of the next generation of transformative treatments
for auto immune
diseases.
BTK is present in the signaling pathways of key innate
and adaptive
cell types of
the immune system.
Being
able to block
or disrupt these signaling
processes can
help in
stopping inflammation and tissue destruction related to autoimmune diseases and
target
some of the underlying pathophysiology.


